Tin oxide nanoislands boost fuel cell performance
Researchers in Japan have revealed that the selective electrodeposition of tin oxide on platinum alloy nanoparticles provides an effective and robust catalyst for Polymer Electrolyte Fuel Cells (PEFCs). These fuel cells could provide an alternative to traditional fossil fuel power, but higher performance and durability under harsh conditions are needed before PEFC vehicles can be considered commercially viable.
Now researchers at the University of Electro-Communications, the University of Tokushima and Japan Synchrotron Radiation Research Institute have synthesised catalysts from platinum cobalt (PtCo3) nanoparticles on carbon (C) with tin oxide (SnO2) nanoislands and shown that they perform better than any previously reported.
Fuel cell research has focused on platinum alloys and transition metal oxides to improve on the durability and catalytic performance of platinum on carbon. Previous work with SnO2 islands grown on platinum tin alloy with carbon had already shown some improvement in the oxygen reduction reactions that occur in fuel cells. However growing islands of only SnO2 on other alloys posed a challenge.
Now, Yasuhiro Iwasawa and his colleagues at the University of Electro-Communications have grown SnO2 islands on Pt3Co nanoparticles on carbon (Pt3Co/C) by selective electrochemical deposition of tin metal, which is then oxidised. The addition of the SnO2 nanoislands doubled the catalytic performance of the Pt3Co/C catalysts. In addition they were undamaged after undergoing 5,000 cycles of voltage changes to test their durability.
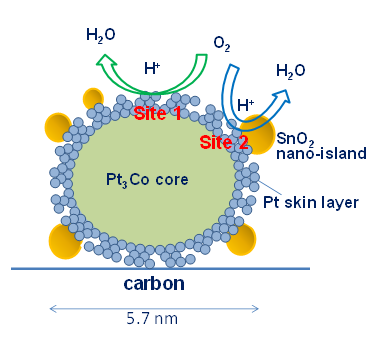
Model structure and proposed oxygen reduction reaction activity sites (sites 1 and 2) for the nano-SnO2-decorated Pt3Co/C with Pt skeleton surface with compressive strain and defects/dislocations.
The structure the Pt3Co nanoparticles form has a Pt3Co core surrounded by a platinum skin that has a rough 'skeleton' morphology. The researchers attribute the high catalytic performance in part to efficient electronic modification specifically at the platinum skin surface, and in particular to the unique property of the SnO2 nanoislands at the compressive platinum skeleton-skin surface.
“In general, adhesion of transition metal oxides on carbon induces depression of the electrical conductivity of the carbon,” explain the researchers in their report. “Hence, the selective nano-SnO2 decoration on the Pt-enriched-surface nanoparticles provides a significant advantage as a cathode catalyst.”





