The opportunities, demands and standards of today’s medical power supply market
The medical power supplies’ global market is rapidly expanding. According to a MarketsandMarkets report ‘Medical Power Supply Market by Technology’, the sector, estimated at $642 million in 2012, is forecast to reach $866.8 million by 2017
Influences include an upsurge in home patient care and a shift towards mobile medical devices. The report identifies Mean Well Enterprises as a major player in this market. Its range of IEC 60601-1 third edition-compliant medical power families comprises open frame, adaptor and enclosed form factors. Sunpower’s access to all Mean Well’s medical products is backed by years of experience and expertise in the market.
The opportunities, though attractive, are restrained by competitive pressures and stringent certification procedures, especially as home-based applications run in environments less-controlled and more demanding than hospital situations.
Medical equipment electrical safety legislation
Equipment regulation authorities have recognised these special challenges, and have generated and revised legislation accordingly. For North America and the EU, IEC/EN 60950 is a set of guidelines for the electrical safety of equipment connected to the public power grid, while IEC/EN 60601-1 is a series of technical standards relating to the safety, essential performance and EMC compatibility of medical electrical devices. These follow the requirements established by IEC/EN 60950 but with increased levels of protection for insulation/isolation, creepage, clearance and leakage current.
As part of this standard, IEC 60601-1-11 – Part 1-11 specifies the basic safety and essential performance of medical electrical equipment and systems used in the home healthcare environment, including external AC/DC power supplies.
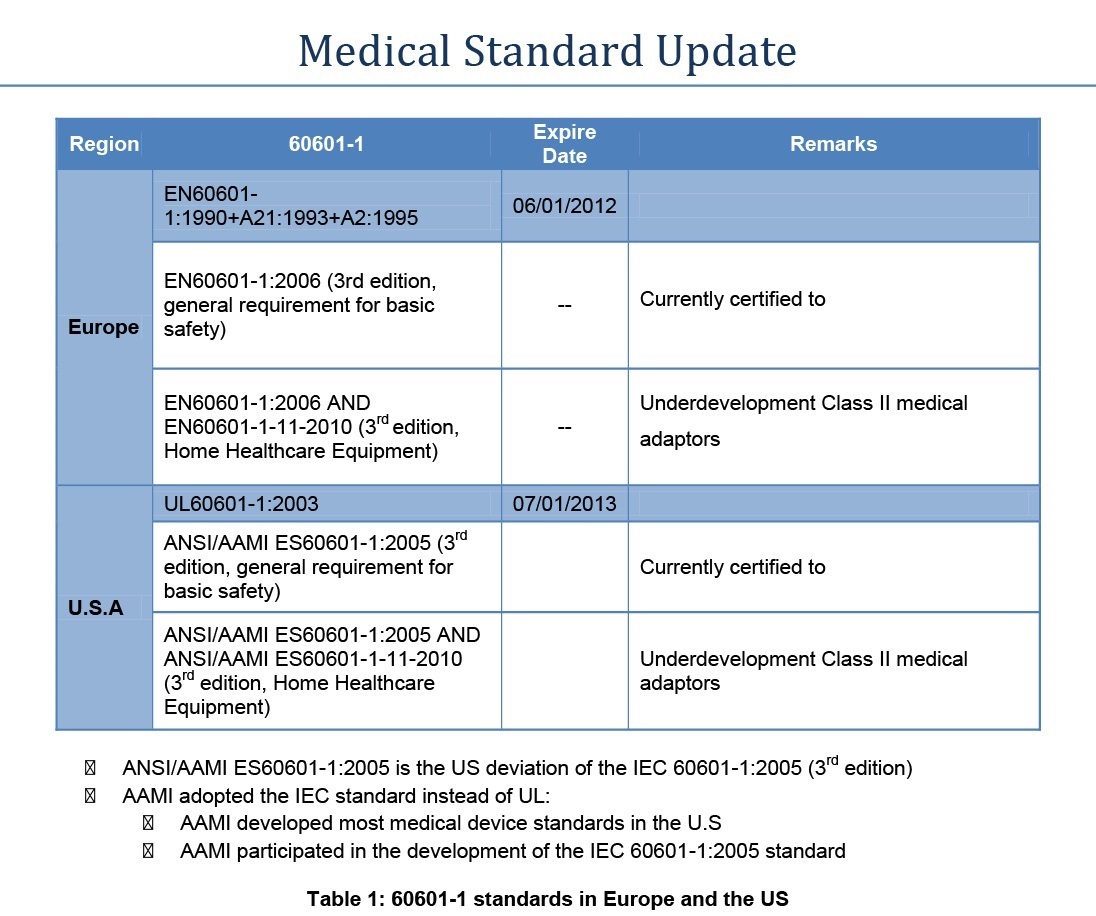
Table 1 below summarises the current status of IEC/EN 60601-1 and 60601-1-11 in Europe and the US.
The standards call for one or more Means of Protection (MOP) within each product. MOPs provide isolation, and can be various combinations of safety insulation, a protective earth, a defined creepage distance, or an air gap or other protective impedance.
MOPP and MOOP
The third, and current edition of IEC/EN 60601-1 differentiates between patient risk and operator risk. A MOP becomes either a MOPP (Means of Patient Protection) or MOOP (Means of Operator Protection). MOPP is creepage and clearance applied specifically to patient contact medical devices. For MOOP, creepage and clearance are identical to IEC60950-1, where the standard is specific to operator contact-only medical devices.
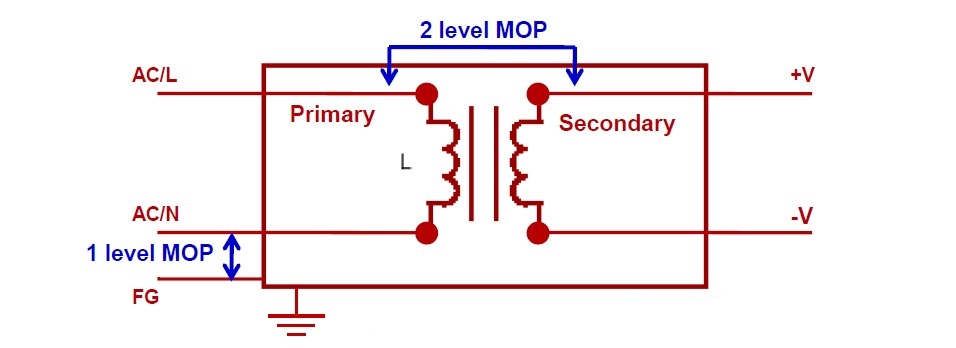
Figure 1: Voltage insulation and MOP levels
It is the device manufacturer’s responsibility to perform a formal risk assessment process (ISO14971) to identify devices likely to make patient contact. Such devices must meet the insulation requirements of MOPP. The insulation must meet at least two MOP between primary to secondary and at least one MOP between primary to protective earth at normal conditions. A power supply that meet two MOPP standards provides the highest level of protection. The standard considers insulation in terms of both creepage and clearance.
Applied parts
While specifying insulation levels essential for medical power supplies and equipment, IEC 60601-1 pays particular attention to applied parts, i.e. the medical device parts that come into physical contact with the patient while the device performs its intended function. Type CF (Cardiac Floating) is the most stringent classification, Type BF (Body Floating) is less so, and Type B is the least stringent.
It is the medical product manufacturer’s responsibility to properly evaluate and determine the classification of applied part types (B/BF/CF) in the final product.
A properly-qualified medical product has suitable applied parts together with power supplies that meet requirements both for maximum leakage current and minimum isolation.
Certifying medical power supplies adequately for their role is essential, yet over-specifying is commercially wasteful. Discussion with a qualified local Mean Well supplier makes sound economic sense. A supplier with experience and expertise in the market can steer customers through certification standards, levels and product choices, to identify the best possible product for an application.










