Safety requirements in medical power supplies
There are specific existing and new requirements for medical safety. Philip Lechner, Avnet Abacus looks at what’s needed for a particular application
When selecting power supplies for medical equipment, there are specific safety requirements and standards that need to be addressed, with new regulations due to be adopted in 2020.
Medical products are classed either as medical devices (MD) or in-vitro diagnostic medical devices (IVD). They have different electrical safety requirements. MDs are defined as products which are connected to, or used in close proximity to patients, such as respirators, syringe pumps and cardiac monitors. IVDs will not normally come into contact with patients and include examples such as blood glucose analysers, centrifuges, and other laboratory equipment.
The medical product manufacturer is responsible for determining how likely a patient is to come into contact with its devices, by carrying out a formal risk assessment process, as defined by ISO14971.
Electrical safety
The first relevant standard to understand is IEC/EN 60950, which is a set of guidelines for the electrical safety of equipment connected to the public power grid, or mains. IEC/EN 60950 will be superseded in December 2020 by IEC/EN 62368. For the purposes of this article, it is appropriate to simply consider them together.
IEC/EN 60601-1 is a further series of standards for the safety and performance of medical electrical devices. IEC/EN 60601-1 is now in its third and fourth edition, and defines different requirements to protect operators and patients. Where there is no significant risk of the medical product coming into contact with a patient, then IEC/EN 60601-1 requirements may not apply and a power supply just conforming to IEC/EN 60950 can often be used.
Means of protection (MOP)
Medical products must incorporate one or more means of protection (MOP) to avoid electrocution. A MOP can be safety insulation, a protective earth, a defined creepage distance, an air gap (clearance) or other protective impedance. These can be used in various combinations – having two MOPs means if one fails, there is another in place.
Creepage is defined as the shortest path between two conductive parts (or between a conductive part and the bounding surface of the equipment) measured along the surface of the insulation, while clearance is the shortest distance between two conductive parts measured through the air. Note also that a protective earth is a fail-safe circuit whose purpose is to protect people from electric shock, while a functional earth is used for EMI suppression.
IEC/EN 60601-1 third and fourth edition differentiate between the risk to patients and the risk to operators. A MOP can therefore be classified as means of patient protection (MOPP) or a means of operator protection (MOOP).
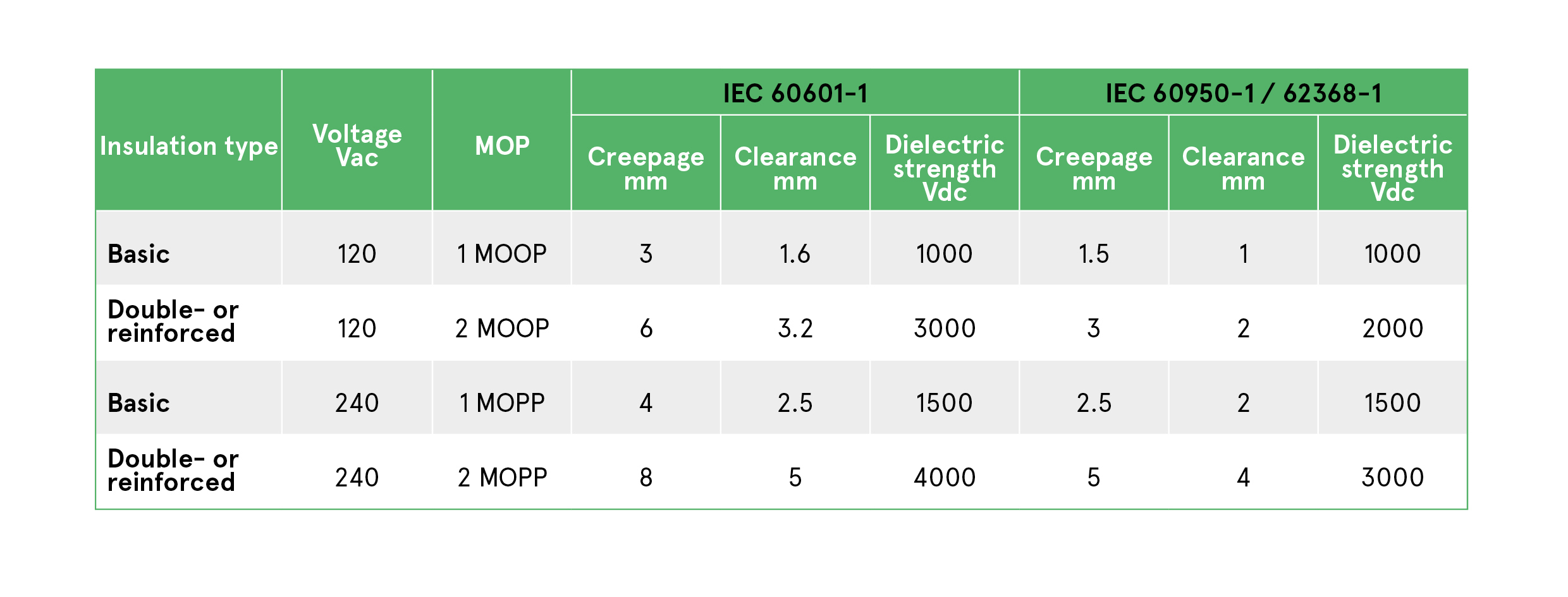
Table 1: IEC/EN 60601-1 requires differing levels of isolation, insulation type, creepage, clearance depending on the MOP level, compared to ITE IEC/EN 60950-1 / 62368-1
The isolation, creepage and insulation requirements for each are set out in Table 1. The main difference between MOOP and MOPP is permissible creepage and clearage. Both requirements are satisfied using basic insulation. To achieve 2 x MOPP or 2 x MOOP qualification, double or reinforced insulation is required, as well as doubled creepage and clearance distances.
Leakage current
In any AC-connected power supply there will be leakage current caused by capacitive coupling across power transformers and by the Y-class filter capacitors that are necessary to maintain EMC performance. In IEC/EN 60601-1 the leakage current limits are unchanged, whether or not there will be patient contact.
For earth discharge current, the maximum earth discharge current normal operation is defined as 0.5mA, while under fault conditions (failure mode 1) it is defined as 1.0mA. For cabinet discharge current, the relevant figures are 0.1mA and 0.5mA for normal and fault conditions, respectively.
In North America, conformance to UL 60601-1 sets the maximum allowable leakage current as 0.3mA, rather than 0.5mA. Where a power supply conforming to IEC/EN 60950 / 62368 is selected to power an IVD, the leakage current limits of IEC/EN 60601-1 must still be met.

One example is a hospital bed. It needs to meet many safety requirements, which are mainly mechanical, such as strengths, gaps and clearances so that patients cannot hurt themselves or fall out. From an electrical point of view, one of the main issues is how to power it: which would normally be either using a built-in AC power supply, or with an external low voltage power supply.
The built-in AC power supply is probably more common because the required power and number of outputs might be higher than an external power supply can supply. The built-in AC supply can also avoid the bulk, weight and vulnerability of an external power supply. However, using a built-in AC supply means the patient might come in contact with conducting parts of the hospital bed, and therefore a 2 x MOPP unit is needed.
An external low voltage power supply, such as 24V, has multiple benefits when the required power is relatively low. As well as removing electrical safety concerns, it also means that the bed does not require annual portable appliance testing (PAT).
The Excelsy CoolX600
The external power supply needs specific features. It must be mechanically strong, with IP4x ingress protection, good strain reliefs, and an appropriate temperature range. Reinforced isolation is required due to the leakage current which results in a 2 x MOPP design. The circuit might be safety Class II but often the protective earth is used only for EMC.
Power supply selection
For IVD applications, MOOP requirements may be met using a power supply that simply conforms to IEC/EN 60950 / 62368. However, it is the responsibility of the medical product manufacturer to demonstrate that the appropriate standard of safety has been achieved.
Power supplies that meet 2 x MOPP standards provide the highest level of protection. When a manufacturer describes a unit as ‘approved for medical applications’ it does not mean that it provides two MOPPs. In general, if a manufacturer has gone to the effort of designing a power supply for 2 x MOPP isolation, it will highlight this on the data sheet. Wherever a more general term is used, such as ‘approved to IEC/EN 60601-1’, the medical device manufacturer must determine what degree of protection the power supply is certified to.
 The LCM1000 from Artesyn
The LCM1000 from Artesyn
If an AC/DC power supply only provides one MOPP, an additional MOP (MOOP or MOPP) may be introduced by including an isolated DC/DC converter. This adds cost and complexity but may be the best solution, particularly where multiple DC rails are required.
For most applications, specifying a 2 x MOPP power supply may be best. Even if there is a small price premium over IEC/EN 60950 / 62368 units, this approach simplifies qualification of the end product. It can also reduce inventory costs by enabling a common power supply to be used in more than one medical product. Recently-introduced AC-DC power supplies are more likely to meet the 2 x MOPP requirement than older designs.
Table 1: IEC/EN 60601-1 requires differing levels of isolation, insulation type, creepage, clearance depending on the MOP level, compared to ITE IEC/EN 60950-1 / 62368-1
Figure 1: Artesyn, Aimtec, Bel Power, Delta, Excelsys and Mean Well offer medical power supplies with 2 x MOPP qualification
https://www.avnet.com/wps/portal/abacus/solutions/technologies/power/