Li-ion battery anode made from Portobello mushrooms
Can portobello mushrooms stop cell phone batteries from degrading over time?
Researchers at the University of California, Riverside Bourns College of Engineering think so. They have created a new type of Li-ion battery anode using portobello mushrooms, which are inexpensive, environmentally friendly and easy to produce.
The current industry standard for rechargeable Li-ion battery anodes is synthetic graphite, which comes with a high cost of manufacturing because it requires tedious purification and preparation processes that are also harmful to the environment.
With the anticipated increase in batteries needed for EVs and electronics, a cheaper and sustainable source to replace graphite is needed. Using biomass, a biological material from living or recently living organisms, as a replacement for graphite, has drawn recent attention because of its high carbon content, low cost and environmental friendliness.
UC Riverside engineers were drawn to using mushrooms as a form of biomass because past research has established they are highly porous, meaning they have a lot of small spaces for liquid or air to pass through. That porosity is important for batteries because it creates more space for the storage and transfer of energy, a critical component to improving battery performance.
In addition, the high potassium salt concentration in mushrooms allows for increased electrolyte-active material over time by activating more pores, gradually increasing its capacity.
A conventional anode allows lithium to fully access most of the material during the first few cycles and capacity fades from electrode damage occurs from that point on. The mushroom carbon anode technology could, with optimisation, replace graphite anodes. It also provides a binderless and current-collector free approach to anode fabrication.
“With battery materials like this, future cell phones may see an increase in run time after many uses, rather than a decrease, due to apparent activation of blind pores within the carbon architectures as the cell charges and discharges over time,” said Brennan Campbell, a graduate student in the Materials Science and Engineering programme at UC Riverside.
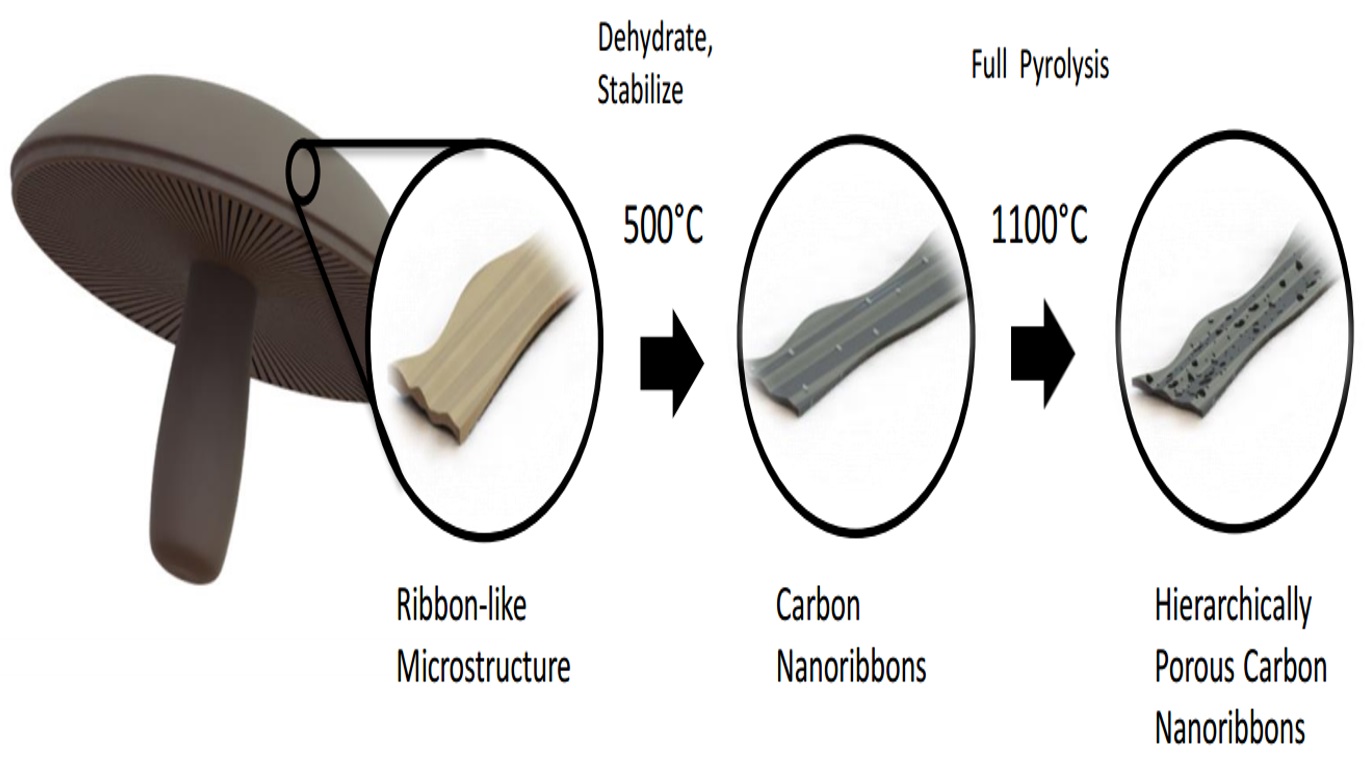
How mushrooms are turned into a material for battery anodes
The research findings were outlined in a paper, Bio-Derived, Binderless, Hierarchically Porous Carbon Anodes for Li-ion Batteries, published in the journal Nature Scientific Reports. It was authored by Cengiz Ozkan and Mihri Ozkan, both professors in the Bourns College of Engineering, and three of their current or former graduate students: Campbell, Robert Ionescu and Zachary Favors.
Campbell and Ionescu have recently formed a start-up company, Ionobell, which is focused on developing cheap, eco-friendly and high-performance batteries for electronics and EVs. They also launched a Kickstarter campaign to raise money for the company.
"Nanocarbon architectures derived from biological materials such as mushrooms can be considered a green and sustainable alternative to graphite-based anodes," said Cengiz Ozkan, a professor of mechanical engineering and materials science and engineering.
The nano-ribbon-like architectures transform upon heat treatment into an interconnected porous network architecture which is important for battery electrodes because such architectures possess a very large surface area for the storage of energy, a critical component to improving battery performance.
"One of the problems with conventional carbons, such as graphite, is that they are typically prepared with chemicals such as acids and activated by bases that are not environmentally friendly," said Mihri Ozkan, a professor of electrical and computer engineering. Therefore, the UC Riverside team is focused on naturally-derived carbons, such as the skin of the caps of portbello mushrooms, for making batteries.
It is expected that nearly 900,000 tons of natural raw graphite would be needed for anode fabrication for nearly six million EV forecast to be built by 2020. This requires that the graphite be treated with harsh chemicals, including hydrofluoric and sulfuric acids, a process that creates large quantities of hazardous waste. The EU projects this process will be unsustainable in the future.










