How to select and apply board-mounted DC/DC converters in medical systems
By Rolf Horn, Applications Engineer at Digi-Key Electronics
With competitive pressure on designers of medical system power supplies to increase power density, reduce footprint, and meet cost constraints, it can be tempting to design their own custom mounted DC/DC converter and fully optimise it for the application.
This article originally appeared in the April'23 magazine issue of Electronic Specifier Design – see ES's Magazine Archives for more featured publications.
Depending upon available resources, this may not be the best option as not only is power supply design challenging, medical DC/DC converters are particularly so since they need to be certified to an array of user and operator safety requirements.
These requirements include IEC/ EN/ES 60601-1 3rd edition for 2x means of patient protection (MOPP) safety and risk management, according to ISO 14971, IPC-A-610 Level 3 criteria for electronic assemblies, and electromagnetic compatibility (EMC) compliance according to IEC 60601-1-2 4th edition. In many instances, a 4:1 input range is needed to operate from various battery and vehicle supplies, and it must have high voltage isolation and low leakage current.
DC/DC converter considerations for medical systems
Industry standards for medical systems design are primarily concerned with safety. The concept of ‘means of protection’ (MOP) is the key to understanding and achieving patient and operator safety. At a minimum, medical devices must include one MOP to protect patients and operators from the risk of an electric shock should a fault occur.
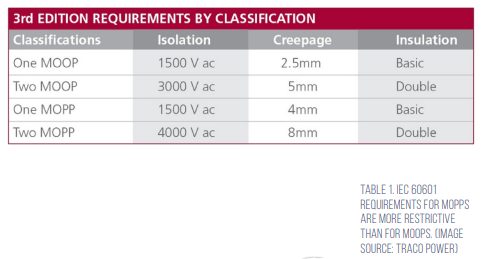
IEC 60601 assigns different MOPs for patients and operators, resulting in specific requirements for MOPP and means of operator protection (MOOP), defined in terms of an isolation voltage, creepage distance, and insulation level (Table 1). MOPP requirements are more restrictive since patients may have less ability to protect themselves and one or two MOPPs or MOOPs may be required by IEC 60601.
The level of protection needed is dependent on the specific application. For example, body floating (BF) safety levels are required for applied parts (AP) that are electrically connected to the patient and must be floating and separated from earth.
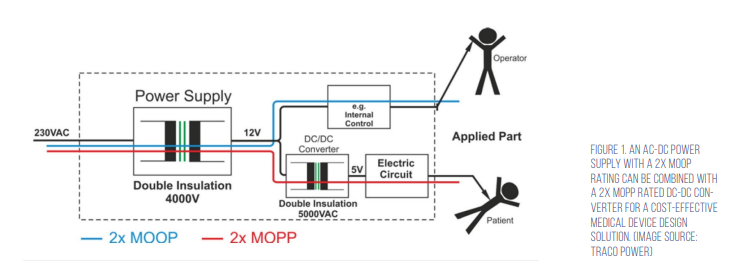
The use of an AC/DC power supply approved for 2x MOPP safety is one approach to meeting IEC 60601, but it may not be the most cost effective. Most ‘medically approved’ AC/DC power supplies are not rated for 2x MOPP and cannot be used in BF applications. In BF medical applications, part of the system needs to meet the less-restrictive 2x MOOP, while the AP section of the system must be rated for BF safety levels and meet 2x MOPP. Combining an AC/DC power supply that meets 2x MOOP with a DC/ DC converter that meets the 2x MOPP is usually the lowest cost solution (Figure 1).
Most off-the-shelf DC/DC converters have isolation ratings of only 500 to 1,600 volts direct current (Vdc) and cannot meet 2x MOPP. Designers can turn to specialised DC/DC converters with up to 5,000 volts alternating current (Vac) isolation, double insulation, and 8mm creepage that meet the 2x MOPP requirements when used with medically approved AC/DC power supplies rated for 2x MOOP.
In addition, standard DC/DC converters have not been subjected to a risk assessment as defined in ISO 14971, which defines the best practices for all life cycle stages of medical devices. This medical device directive also requires manufacturers of DC/DC converters and other medically approved devices to implement an ISO 13485-compliant quality management system.
Protecting system operation
Ensuring system operation is another requirement for medical devices. The printed circuit boards (PC boards) in medical devices must meet the requirements of IPC-A-610 Level 3, Class 3 for high performance products. Class 3 PC boards are expected to provide continuous performance, or performance on demand, with no equipment downtime.
Electromagnetic compatibility (EMC) requirements for medical designs are strict and have recently become even more demanding. IEC 60601-1-2:2014+A1:2020 applies to the safety and performance of medical devices and systems in the presence of electromagnetic disturbances. It also limits the electromagnetic disturbances emitted by medical devices and systems. In the latest edition, published in 2020, conducted emissions (CISPR 11) must be tested at minimum and maximum rated voltage, compared with the single voltage test used in the previous edition.
Medical devices and systems such as DC/ DC converters that were able to pass the single voltage test may fail when tested at minimum and maximum rated voltages.
Other changes in the latest edition include:
- Immunity test levels are now specified relative to the environment of intended use, and the location categories are harmonised with IEC 60601-1-11
- Immunity tests and test levels are specified based on the ports in medical electrical equipment within a medical electrical system
- Additional tests have been included to ensure the safe operation of medical devices and systems when portable communications devices are used in close proximity
- Standard DC/DC converters for medical applications
When tasked with meeting the myriad of medical safety and performance requirements, designers can either spend time and resources developing their own converter and getting it through the qualification and certification processes or turn to the THM 60WI series from Traco Power. These 60W DC/DC converters come in a 2.3 x 1.45”, quarter-brick plastic package and feature a wide 4:1 input voltage range making them suitable for both AC powered and battery-powered designs. They have 5,000 VAC reinforced isolation between the input and output, a leakage current of less than 4.5 microamperes (μA), are approved to IEC/EN/ES 60601-1 3rd edition for 2 x MOPP, IEC/EN/UL 62368-1, and have an ISO 14971 risk management file. Their design and production meet the quality management system requirements of ISO 13485.
The THM 60WI series comprises 12 models with input ranges of 9 to 36VDC or 18 to 75VDC, and single or dual outputs of 5.1, 12, 15, 24, ±12 volts, or ±15VDC with up to 92% efficiency. For example, the model THM 60-2411WI has an input voltage range of 9 to 36VDC, an output of 5.1VDC at 12A, and an efficiency of 90%. This series of 2 x MOPP compliant and BF rated DC/DC converters are suited for AP applications. They have a calculated MTBF of over one million hours (according to MIL-HDBK-217F, ground benign) and have a five-year warranty.
A summary of features include:
- IEC 60601-1-2 4th edition EMC compliance
- 5,000 Vac reinforced isolation with less than 4.5µA leakage current
- Remote sense; output voltage trim and remote on/off functions
- Protection against undervoltage on the input, output short-circuit, overtemperature, and output overvoltage
- An ambient operating temperature range of –40°C to +75°C that can be extended using an optional heatsink
- Thermal design options
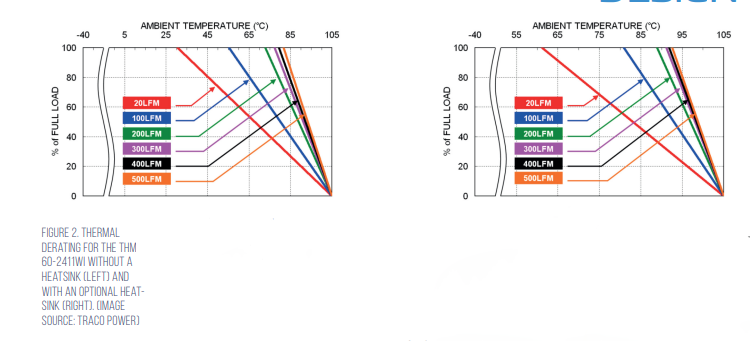
The THM 60WI series is specified for an ambient temperature up to +75°C, with derating. For more demanding thermal environments, Traco also offers the THM-HS1 heatsink with a thermal impedance of 4.71 Kelvin/watt (K/W) that significantly increases thermal dissipation under both natural convection and forced-air conditions. For example, when used with the THM 60-2411WI, the THM-HS1 extends the maximum full-load operating temperature from about 30°C to 60°C (with 20 linear feet per minute (LFM) of airflow), and from about 80°C to 90°C (with 500 LFM airflow) (Figure 2).
EN 55032 compliance
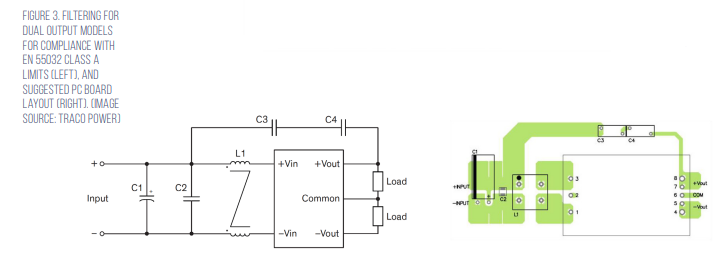
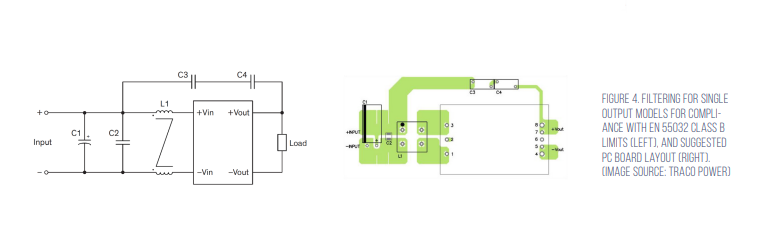
Under EN 55032 in North America, any equipment primarily used in a residential environment must meet Class B limits. All other equipment must comply with Class A limits. Traco offers suggested electromagnetic interference (EMI) filter implementations for both Class A and Class B environments (Figure 3).
The Class A filter consists of; C1, 100 microfarad (μF)/100 volt aluminum capacitor; C2, 2.2 μF/100 volt 1210 multilayer ceramic capacitor (MLCC); C3 and C4, 100 picofarad (pF) Y1 capacitors; L1, 285 microhenry (μH) common-mode choke (TCK-103 from Traco Power).
The suggested Class B EMI filter for Class B is shown in Figure 4. It consists of; C1, 100 μF/100 volt aluminum capacitor; C2, C3, and C4, 2.2 μF/100 volt, 1210 MLCCs; C5 and C6, 47 pF Y1 capacitors; C7 and C8, 33 pF Y1 capacitors; L1 and L2, 285 μH common mode chokes (TCK-103) (Figure 4).
Conclusion
Power supply design for medical applications is challenging, but designers can opt for off-the-shelf board-mounted DC/DC converters. As shown, the right DC/DC converter can help enhance all aspects of medical device safety. It can also support the use of a variety of powering architectures including AC mains and battery-powered solutions, while providing overvoltage, short-circuit, and undervoltage protection.