Enzymes point the way toward self-powered fluidic devices
Researchers at the University of Pittsburgh's Swanson School of Engineering, along with collaborators at Penn State University's Chemistry Department, have discovered a novel way of utilising the chemical reactions of certain enzymes to trigger self-powered mechanical movement. Their research, "Convective flow reversal in self-powered enzyme micropumps," was published this week in the journal Proceedings of the National Academy of Sciences.
The computational modeling developed by Anna C. Balazs, Distinguished Professor of Chemical and Petroleum Engineering at Pitt with post-doctoral associate Henry Shum, and experiments conducted by Ayusman Sen, Distinguished Professor of Chemistry at Penn State University, along with graduate student Isamar Ortiz, revealed that simple enzymatic reactions can drive complex, time-dependent flows.
Dr. Balazs says: "Small-scale chemical synthesis and analysis commonly occur in fluid-filled chambers and require monitoring and intervention. Ideally, you would prefer that the process be as autonomous as possible." According to Dr. Sen who made the initial discovery of enzyme pumps: "These pumps provide precise control over flow rate without the aid of an external power source and are capable of turning on in response to specific chemicals in solution. Hence, the surface-bound enzymes can be used to combine sensing and fluidic pumping into a single self-powered microdevice."
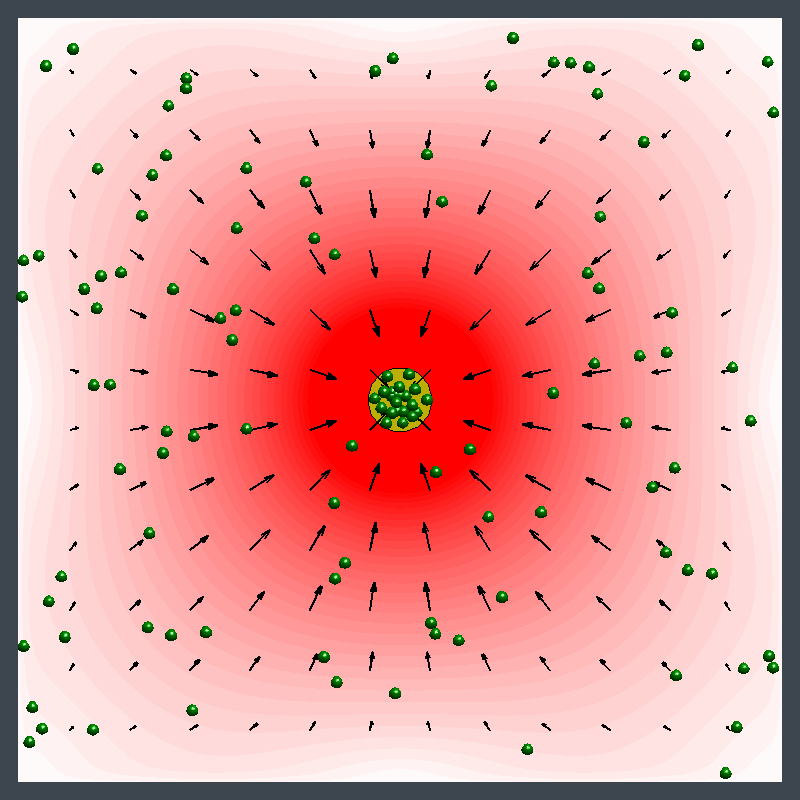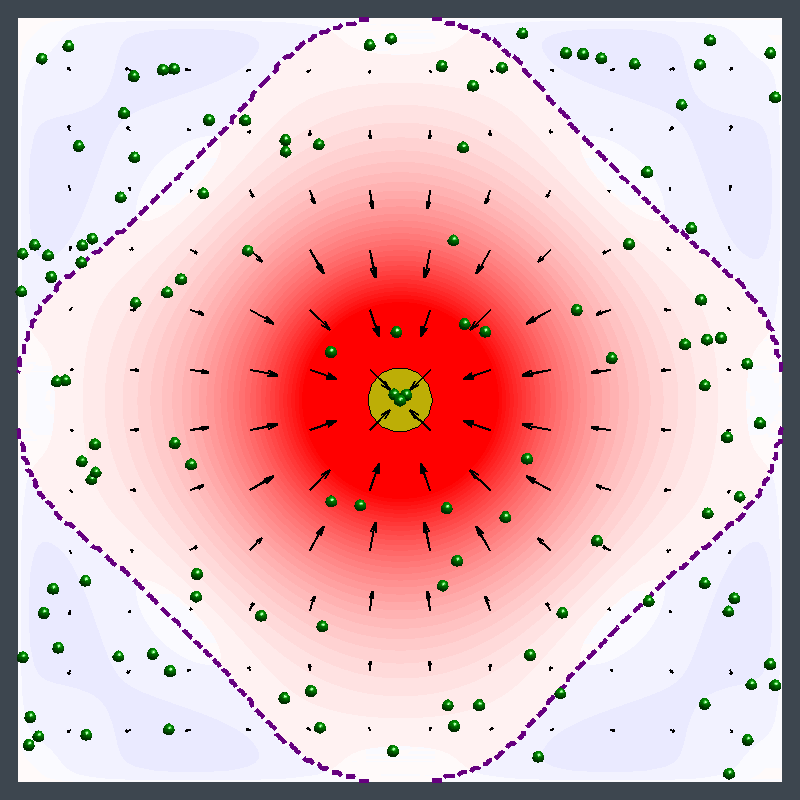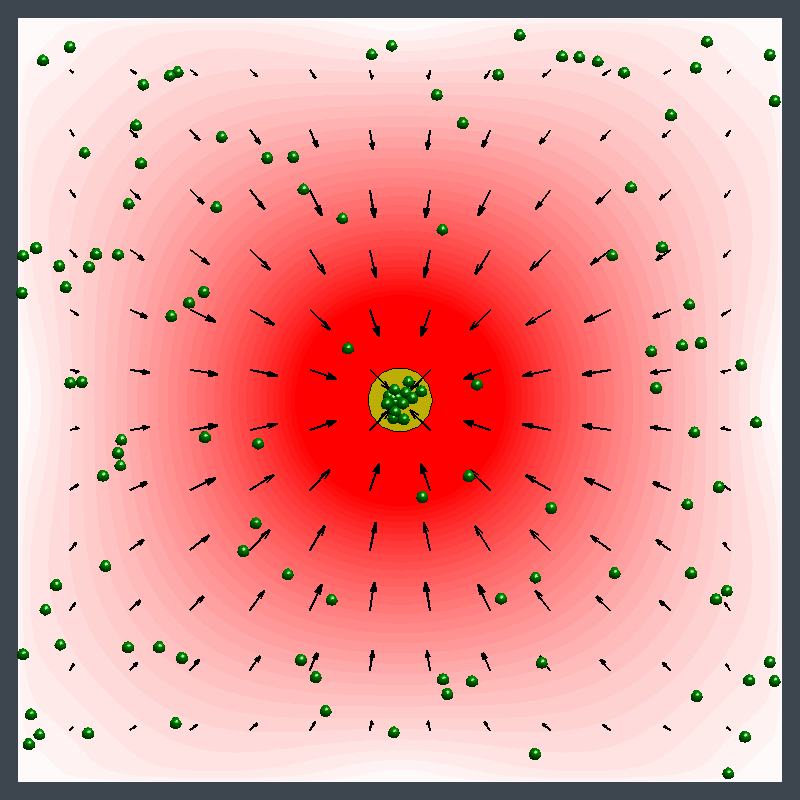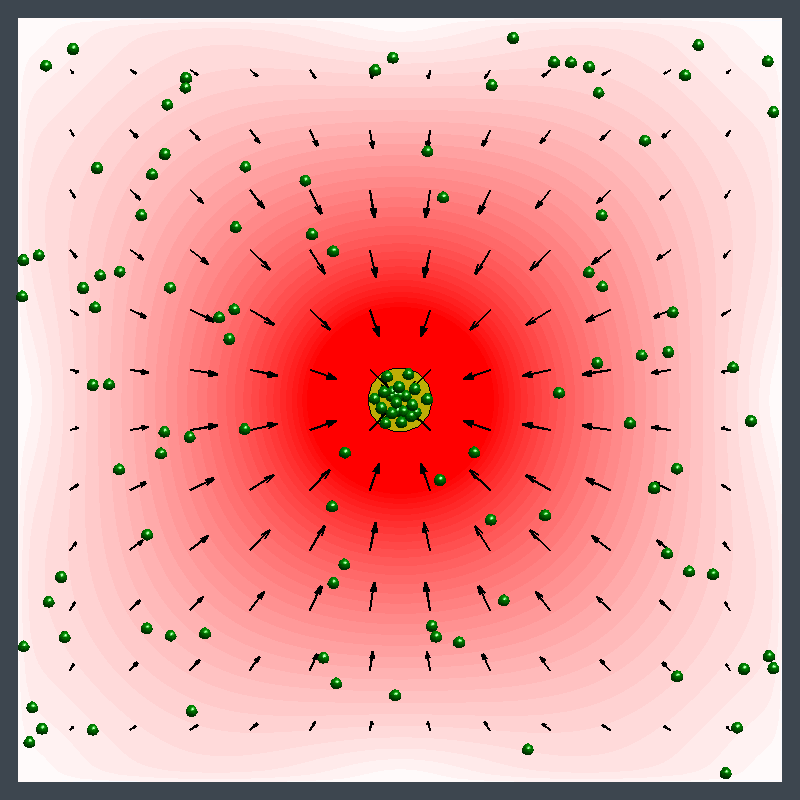
Dr. Shum examined the factors that cause variations in density in the enzymatic reactions and developed a mathematical model. The model pinpointed where the relative density and relative diffusivity of the reagents and products controls the behavior, enabling him to map a parameter range where the fluid could move in different directions at different times.
"The reaction causes a change in the density of the fluid, leading to a fluid flow," Dr. Shum explained. "It creates a chemo-mechanical transduction, which is a beautiful example of chemical energy creating mechanical action, much in the same way that the human body converts chemical energy from food into movement."
Ms. Ortiz's experiments at Penn State with urease enzyme agree with the Pitt simulations. Unlike other enzymes, which always pumped fluid in the same direction, urease generated an unexpected flow pattern, with the direction of flow changing over space and time. "It's like hot air rising above a burning candle," Ms. Ortiz explained. "With urease, it's as if the air around the candle sinks for a finite time before starting to rise."
Even more complex behavior could be built up using different enzymes placed at different locations to create a dynamic cascade of events. The resulting chemical reaction network is analogous to an electrical system in a computer. Since each enzyme will behave differently, a multistage chemical reaction could be "programmed" into an experiment, with each step generating flow as determined by the action of a specific enzyme.
"Much like Disney's "The Sorcerer's Apprentice," where you could snap your fingers and have mops and brooms clean your room for you, it's a scientist's wish to create systems which behave autonomously and controllably," Dr. Balazs said. As Ms. Ortiz points out: "The self-powered enzyme pumps could be used as self-regulated, stimuli-responsive, active, delivery vehicles."





