Accelerating clinical trials using machine learning
This past 12 months has been something of a challenge for us all – with everyone essentially living in limbo waiting for medical research to discover a treatment or prevention strategy for a brand-new disease. At the recent CES show, Charles Fisher, CEO, Unlearn.AI, discussed the role of machine learning in bringing cures for diseases such as COVID-19 to the market faster.
As the new coronavirus vaccines are rolling-out over the coming months we all hope that we will be able to get back to some semblance of normality sooner rather than later. However, even in a post-COVID world, there are still going to be millions of people with incurable or chronic diseases, who will still be waiting for treatments that will change their lives.
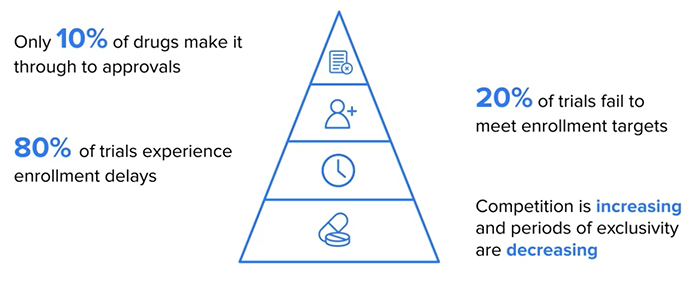
One of the biggest challenges to getting new treatments to these patients who need them quickly, is running human clinical trials to demonstrate that these treatments are safe and effective. These clinical trials are incredibly expensive and risky, and only around ten percent of drugs make it through the trial process.
Above: Clinical trials are too slow, risky, and expensive
It is also a significantly time-consuming process and, in many cases, take five to ten years to demonstrate that a treatment is effective, with 80% of clinical trials experiencing enrolment delays. All the while patients who need those drugs are waiting expectantly for them, living unfulfilled and impaired lives, often in pain and uncertainty.
Fisher commented: “At Unlearn.AI we use artificial intelligence to answer the question of how biopharma companies can run these clinical trials in half the time. And doing that we can decrease the size of trials while retaining the statistical power we need to know if drugs are safe and effective, running them much more efficiently.
“We can also increase the statistical power in ongoing clinical trials to really understand and see whether or not drugs are actually safe and effective. And by reducing variability, we can develop better and more accurate decisions around whether or not drugs are to be approved.”
Digital twins
A standard clinical trial takes a real patient and gives them a brand-new therapy, often that’s never been given before. The question the trial is asking is how different would that patient’s outcome have been if they had received the current standard of care for their condition, usually in combination with a placebo?
The difference between the two provides the insight into whether that treatment is effective. Using AI and digital twins, even if there is no data because a treatment is brand-new, Unlearn.AI can collect data from 10,000s, maybe even 100,000s of patients, who have already received the standard of care, either in previously run clinical trials, or just in the real-world when visiting their physician.

Above: Our AI-driven trials will generate the strongest evidence while accelerating clinical research
Fisher continued: “We can leverage this data with new artificial intelligence technologies to predict how patients in clinical trials will respond if they are randomised to control groups, and then use that information to reduce the number of patients we need to enrol in these clinical trials, by reducing the number of patients we need to actually get placebos.
“We train these artificial intelligence models using methods from an area of machine learning known as deep learning, to create simulations of patients, where we can create simulated medical records that tell us how a particular patient would respond in a clinical trial if they were to receive a placebo in the standard of care.
“And then by giving that real patient the actual investigational treatment and subtracting what would be predicted if they were to receive a placebo, we can obtain an estimate for the treatment effect. By doing this we can effectively eliminate half of the patients in the clinical trial.”
Examples
Historical data from electronic health records and previous clinical trials, can be leveraged with machine learning, to enable more confident results from clinical trials with digital twins. As an example, Fisher cited a randomised controlled trial - the gold standard for telling if a new treatment is safe and effective.
When a patient enrols in that trial, before they are given their first treatment, Unlearn.AI can collect a swathe of data from biomarkers, genetic data or blood tests that inform their disease state, and input that into an artificial intelligence model and create a digital twin.
That patient can then go on to be randomised to receive the after treatment or the control. Then sophisticated statistical analysis methods can be deployed to obtain very confident inferences about whether or not that treatment is effective. And so the digital twin can decrease the number of subjects needed to obtain a confident estimate from that trial.
“In certain cases, we can even go farther than that,” Fisher continued. “So for pivotal trials, such as a phase three clinical trial, we need to ensure that we can provide statistical power and efficiency without introducing any bias, and we can do that with these statistical methods.
“However, for earlier stage trials, things that would be phase two or proof of concept studies, we can eliminate control arms entirely, creating what we call an intelligent control arm of simulated patients on placebo. Thereby, running a clinical trial that includes half as many patients, produces just as good results, and in half the time.”
Fisher emphasised that while these methods of using AI and deep learning to create simulated placebo patients for clinical trials, may sound like science fiction, it is rapidly being turned into science fact. “We are using methods that are supported by existing regulatory guidance from the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA), and we have had our own interactions with the FDA and are continuing to work with regulatory agencies to define pathways forward,” he added.
Fisher concluded: “We’re really looking forward to partnering with pharmaceutical companies to help take those patients with chronic and incurable diseases who are in limbo and get them the therapies they need as quickly as possible.”







